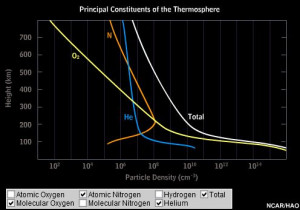
Principal Constituents of the Thermosphere
The gases in the lower layers of Earth's atmosphere (the troposphere, stratosphere, and mesosphere) are well-mixed, and the density of each different type of gas (nitrogen, oxygen, etc.) declines at about the same rate with increasing altitude. However, in the extremely thin air of the thermosphere the mixture of gases no longer behaves as much like a well-mixed fluid as it does in the denser, lower atmosphere. The physics of particle (molecules and atoms of gases) motion begins to play an increasingly important role in governing the behavior of the constituents of the thermosphere. This means that the density of each constituent varies at a different rate as altitude increases.
The graph below shows density of six important constituents of the thermosphere. Click on the check boxes at the bottom of the graph to hide or show individual curves. (If you don't see the graph, you may need to install the latest version of the Flash player onto your computer.)
The thermosphere contains significant numbers of individual atoms of oxygen and nitrogen as well as the molecular (two atoms chemically bound together) oxygen and nitrogen (O2 and N2) that is common in the lower atmosphere. Energy in the form of ultraviolet (UV) radiation from the Sun breaks the bonds of some of the oxygen and nitrogen molecules to generate the supplies of atomic oxygen and nitrogen. This process is called photo-dissociation.
The density of heavier particles (such as molecules of nitrogen and oxygen) falls off more rapidly as altitude increases than does the density of lighter particles (such as atoms of nitrogen and oxygen). Hydrogen and helium, which are lighter than even atomic oxygen and nitrogen, become relatively abundant in the uppermost reaches of the thermosphere.